Units Of Gibbs Free Energy Change
G H - TS. 3 Standard absolute entropy.

The Standard Free Energy Change Of A Reaction Is Deltag Kj
Worked Example of Gibbs Free Energy Calculation 1 Kelvin K is the SI.

Units of gibbs free energy change. We have an equation. G H - TS If the reaction is run at constant temperature this equation can be written as follows. Willard Gibbs 1873 available energy free energy graph which shows a plane perpendicular to the axis of v and passing through point A which represents the initial state of the bodyMN is the section of the surface of dissipated energyQε and Qη are sections of the planes η 0 and ε 0 and therefore parallel to the axes of ε internal energy and η respectively.
DG DH - TDS For a spontaneous process at constant temperature and pressure DG must be negative. The first free energy change at 25C is accurate because the tabulated values of enthalpies of formation and entropies are valid at that standard temperature. Given ΔH and S are -815KJ and -1890JK.
S entropy Gibbs free energy is a state function hence it doesnt depend on the path. N 2 3H 2 2NH 3. So it is necessary to convert the units - usually by dividing the entropy values by 1000 so that they are measured in kJ K-1 mol-1.
The Δ G f values given above for enstatite are both negative. Chemists normally measure energy both enthalpy and Gibbs free energy in kJ mol-1 kilojoules per mole but measure entropy in J K-1 mol-1 joules per kelvin per mole. Combine the standard enthalpy of formation and the standard entropy of a substance to get its standard free energy of formation.
Δ G 566 673 0173 450 kJ. Gibbs free energy denoted G combines enthalpy and entropy into a single value. Use the data in Table P2 to calculate ΔGo for the reduction of ferric ion by iodide.
Delta G can predict the direction of the chemical reaction under two conditions. The SI unit for Gibbs energy is the kilojoule. The free energy change DG is equal to -TDSuniv and it applies just to a system itself without regard for the surroundings.
Changes in the Gibbs free energy G correspond to changes in free energy for processes at constant temperature and pressure. The change in free energy Delta G is equal to the sum of the enthalpy plus the product of the temperature and entropy of the system. It is defined by the Gibbs equation.
Thus ΔGo is 156 kJ for the reaction as written and the reaction is spontaneous. Have this reaction here where if I had a mole of methane and I react that with two moles of oxygen Ill produce the mole of carbon dioxide and two moles of water but we want to answer in this video is whether this reaction is spontaneous and we learned in the last video that to answer that question we have to turn to Gibbs free energy or the change in Gibbs free energy and the change in Gibbs. Determine the standard free energy change for the following reaction at 25 o C.
Delta H ΔH is the enthalpy change in kilojoules per mole KJmole the temperature is measured in Kelvin and the entropy change is measured in joules per kelvin per mole. The change in the Gibbs free energy of the system that occurs during a reaction is therefore equal to the change in the enthalpy of the system minus the change in the product of the temperature times the entropy of the system. The Gibbs Free Energy of Formation for enstatite from oxides MgO and SiO 2 Δ G f enstatite oxides is about -354 Jmole at room temperature and pressure.
Click to see full answer. Summary of Gibbs Free Energy. ΔG ΔH TΔS.
If temperature is given in other units such as C or F you will. Substitute the above values in this equation. ΔG nFE cell 6 mole96 485 J V mol027 V 156 104 J 156 kJ mol Cr2O2 7.
ΔG ΔH - TΔS ΔG -8904 - 298-02442 -8176 kJ mol-1 It is easy as long as you remember to convert the entropy change value into kJ. ΔG -815KJ 298 K -01890KJK ΔG -247KJ. ΔG ΔH TΔS.
So change in Gibbs free energy is equal to the change in enthalpy minus the product of temperature and entropy change of the system. 2 The standard enthalpy change for a reaction is also referred to as the standard heat of reaction. The two results for the free energy of reaction differ because of the change in temperature.
ΔGrxn ΣΔG fproductsΣΔG freactants Δ G rxn Σ Δ G f products Σ Δ G f reactants can be used to determine standard free energy change of a reaction. Gibbs Energy values are most often today given in units of joulesmole or less commonly caloriesmole. So if you had to calculate the Gibbs free energy change at say 298 K you can just slot the numbers in.
Standard Free Energy Equation
ΔG ΔH TΔS 4501 kJ 298 K 1188JK 1 kJ 1000 J 4501 kJ 354 kJ 96 kJ At 298 K 25 C ΔG 0 so boiling is nonspontaneous not spontaneous. The term D G provides us with a value for the maximal work we could obtain.
For a reversible process that does not involve external work we can express the change in free energy in terms of volume pressure entropy and temperature thereby eliminating ΔH from the equation for ΔG.
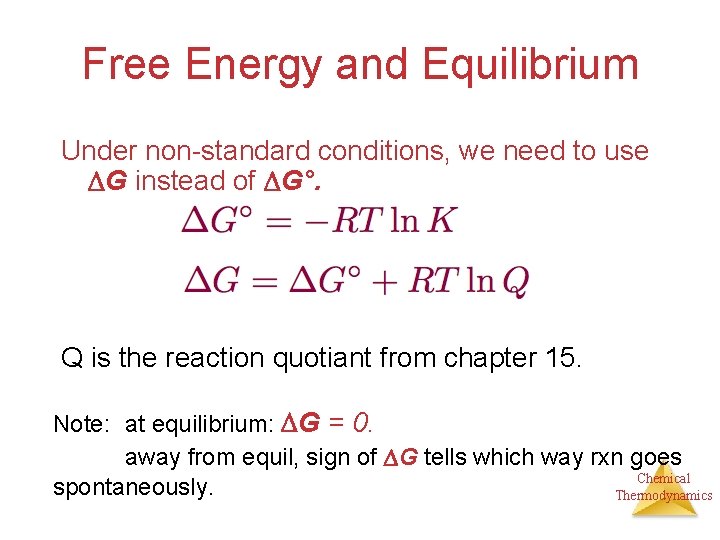
Standard free energy equation. May be interpreted as representing the difference between the energy produced by the process Δ H and the energy lost to the surroundings T Δ S. Free Energy ΔG is equal to the maximum amount of work a system can perform on its surroundings while undergoing a spontaneous change. Keq Equilibrium Constant.
ΔG ΔGo RTlnQ ΔG free energy at any moment ΔGo standard-state free energy. If we know the equilibrium constant K eq for a chemical change or if we can determine the equilibrium constant we can calculate the standard state free energy change G o for the reaction using the equation. Using Cell Potentials to.
ΔG ΔH - TΔS Thats all you need to know. ΔG ΔH TΔS For simplicitys sake the subscript sys will be omitted henceforth. The difference between the energy produced and the energy lost is the energy available or free to do useful work by the process Δ G.
Gibbs free energy equation. In this equation R 8314 J mol-1 K-1 or 0008314 kJ mol-1 K-1. Standard Free Energy Change and Equilibrium Constant Calculator.
Standard Free Energy Change and Equilibrium Constant Calculator. The relationship between the free energy of reaction at any moment in time G and the standard-state free energy of reaction G o is described by the following equation. R Universal Gas Constant.
Substitution into the standard free energy equation yields. This is how standard Gibbs free energy change is calculated. In this equation the term D G o provides us with a value for the maximal work we could obtain from the reaction starting with all reactants and products in their standard states and going to equilibrium.
When a system changes from an initial state to a final state the Gibbs free energy ΔG equals the work exchanged by the system with its surroundings minus the work of the pressure force. The difference between standard free energy change and the actual free energy change for a particular set of conditions. The Gibbs free energy equation is dependent on pressure.
ΔGo Standard Free Energy Change. If we know the standard state free energy change Gofor a chemical process at some temperature T we can calculate the equilibriumconstant for the process at that temperature using the relationship between Goand K. LatexDelta Gcirc rxn Sigma Delta G_ fcirc products-Sigma Delta G_ fcirc reactants latex As with standard heats of formation the standard free energy of a substance represents the free energy change associated with the formation of the substance from the elements in their most stable forms as they exist under the standard.
J kJ kcal erg. T is the temperature on the Kelvin scale. The standard Gibbs free energy of the reaction can also be determined according to.
Look up the Standard Free Energy of Formation of H2O g and multiply by its coefficient 2 in the equation. 2 moles -2372 kjmole -4744 kj Standard Free Energy of Formation for two moles H2O l Look for the Standard Free Energy of Formation of CO2g and multiply by its coefficient 4. Free energy is a state function and at constant temperature and pressure the standard free energy change ΔG may be expressed as the following.
The standard free energy is then Δ G n F E cell Δ G 2 96485 C mol 1247 J C 2406 kJ mol The reaction is spontaneous as indicated by a negative free energy change and a. G G o RT ln Q In this equation R is the ideal gas constant in units of Jmol-K T is the temperature in kelvin ln represents a logarithm to the base e and Q is the reaction quotient at that moment in time. R 8314 J mol-1K-1or 0008314 kJ mol-1K-1.
The free energy as defined by. The following equation relates the standard-state free energy of reaction with the free energy at any point in a given reaction not necessarily at standard-state conditions. K eq is the equilibrium constant at the temperature T.

