G H - TS. 3 Standard absolute entropy.

The Standard Free Energy Change Of A Reaction Is Deltag Kj
Worked Example of Gibbs Free Energy Calculation 1 Kelvin K is the SI.

Units of gibbs free energy change. We have an equation. G H - TS If the reaction is run at constant temperature this equation can be written as follows. Willard Gibbs 1873 available energy free energy graph which shows a plane perpendicular to the axis of v and passing through point A which represents the initial state of the bodyMN is the section of the surface of dissipated energyQε and Qη are sections of the planes η 0 and ε 0 and therefore parallel to the axes of ε internal energy and η respectively.
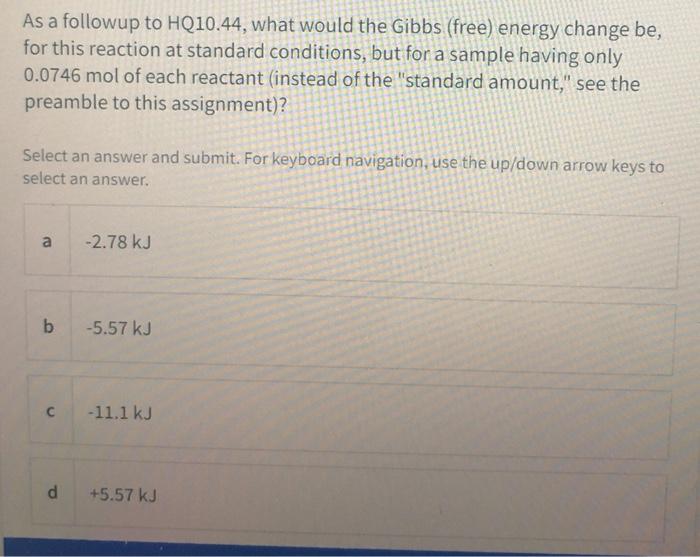
DG DH - TDS For a spontaneous process at constant temperature and pressure DG must be negative. The first free energy change at 25C is accurate because the tabulated values of enthalpies of formation and entropies are valid at that standard temperature. Given ΔH and S are -815KJ and -1890JK.
S entropy Gibbs free energy is a state function hence it doesnt depend on the path. N 2 3H 2 2NH 3. So it is necessary to convert the units - usually by dividing the entropy values by 1000 so that they are measured in kJ K-1 mol-1.
The Δ G f values given above for enstatite are both negative. Chemists normally measure energy both enthalpy and Gibbs free energy in kJ mol-1 kilojoules per mole but measure entropy in J K-1 mol-1 joules per kelvin per mole. Combine the standard enthalpy of formation and the standard entropy of a substance to get its standard free energy of formation.
Δ G 566 673 0173 450 kJ. Gibbs free energy denoted G combines enthalpy and entropy into a single value. Use the data in Table P2 to calculate ΔGo for the reduction of ferric ion by iodide.
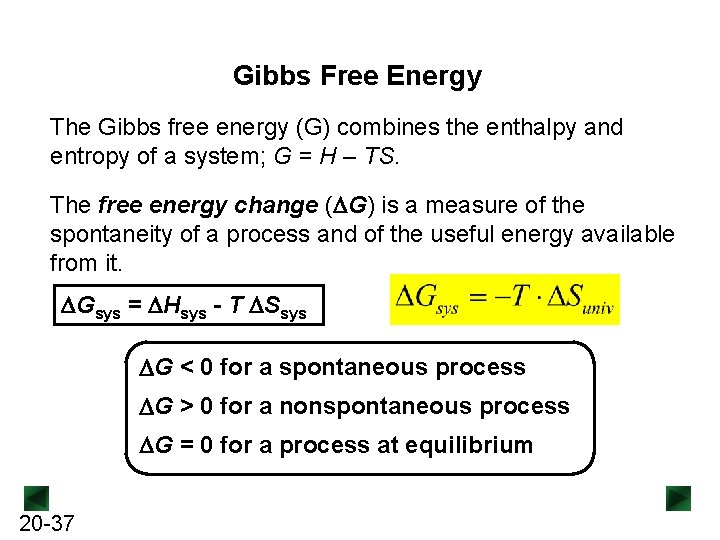
Delta G can predict the direction of the chemical reaction under two conditions. The SI unit for Gibbs energy is the kilojoule. The free energy change DG is equal to -TDSuniv and it applies just to a system itself without regard for the surroundings.
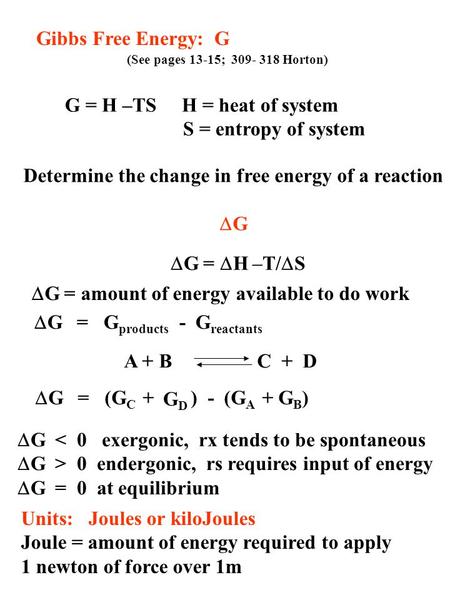
Changes in the Gibbs free energy G correspond to changes in free energy for processes at constant temperature and pressure. The change in free energy Delta G is equal to the sum of the enthalpy plus the product of the temperature and entropy of the system. It is defined by the Gibbs equation.
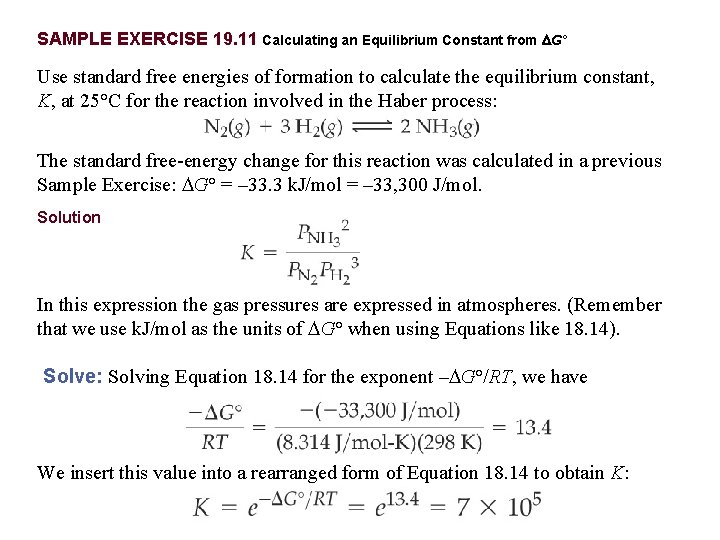
Thus ΔGo is 156 kJ for the reaction as written and the reaction is spontaneous. Have this reaction here where if I had a mole of methane and I react that with two moles of oxygen Ill produce the mole of carbon dioxide and two moles of water but we want to answer in this video is whether this reaction is spontaneous and we learned in the last video that to answer that question we have to turn to Gibbs free energy or the change in Gibbs free energy and the change in Gibbs. Determine the standard free energy change for the following reaction at 25 o C.
Delta H ΔH is the enthalpy change in kilojoules per mole KJmole the temperature is measured in Kelvin and the entropy change is measured in joules per kelvin per mole. The change in the Gibbs free energy of the system that occurs during a reaction is therefore equal to the change in the enthalpy of the system minus the change in the product of the temperature times the entropy of the system. The Gibbs Free Energy of Formation for enstatite from oxides MgO and SiO 2 Δ G f enstatite oxides is about -354 Jmole at room temperature and pressure.
Click to see full answer. Summary of Gibbs Free Energy. ΔG ΔH TΔS.
If temperature is given in other units such as C or F you will. Substitute the above values in this equation. ΔG nFE cell 6 mole96 485 J V mol027 V 156 104 J 156 kJ mol Cr2O2 7.
ΔG ΔH - TΔS ΔG -8904 - 298-02442 -8176 kJ mol-1 It is easy as long as you remember to convert the entropy change value into kJ. ΔG -815KJ 298 K -01890KJK ΔG -247KJ. ΔG ΔH TΔS.
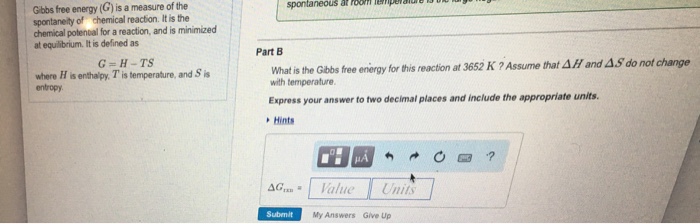
So change in Gibbs free energy is equal to the change in enthalpy minus the product of temperature and entropy change of the system. 2 The standard enthalpy change for a reaction is also referred to as the standard heat of reaction. The two results for the free energy of reaction differ because of the change in temperature.
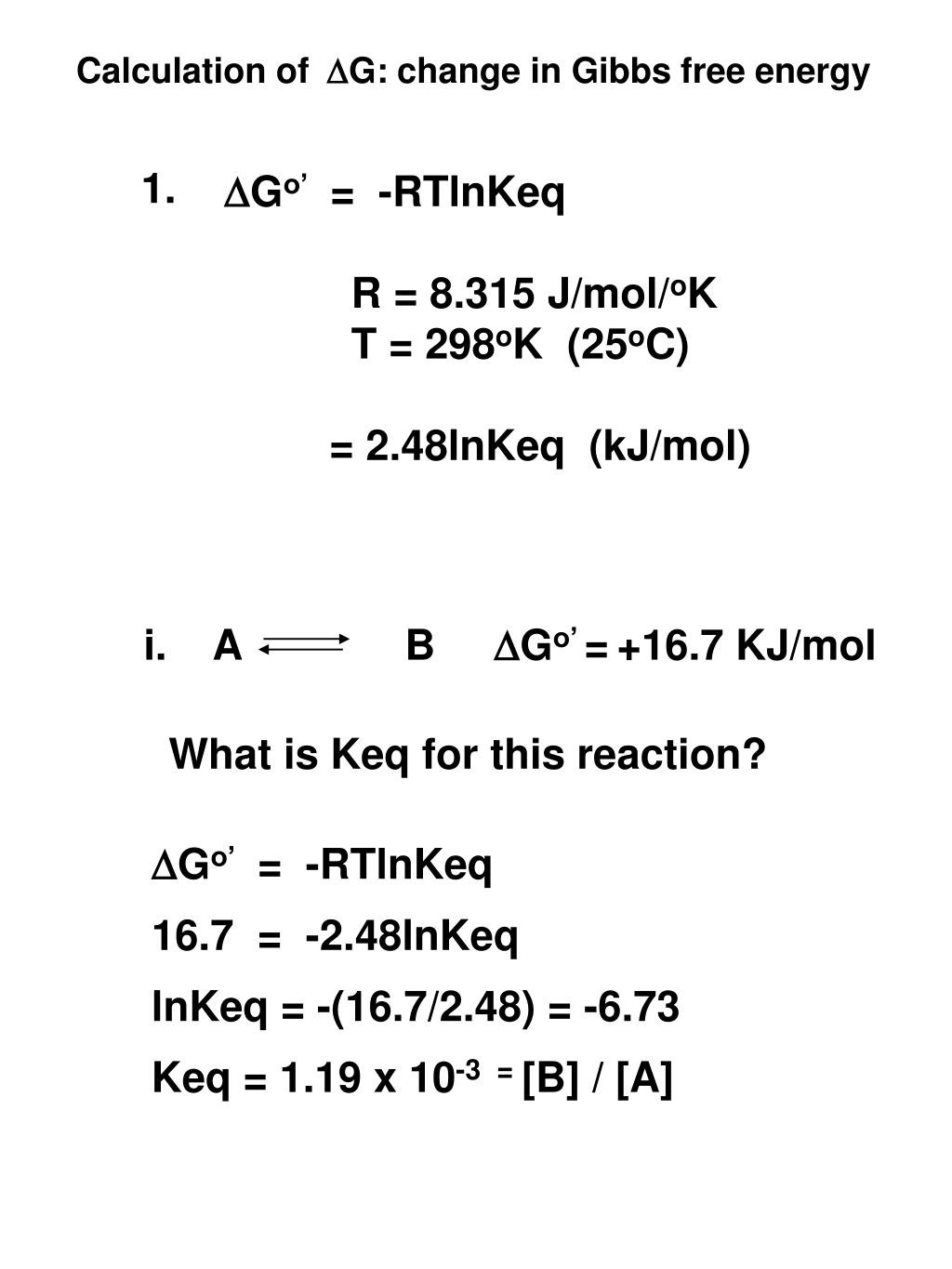
ΔGrxn ΣΔG fproductsΣΔG freactants Δ G rxn Σ Δ G f products Σ Δ G f reactants can be used to determine standard free energy change of a reaction. Gibbs Energy values are most often today given in units of joulesmole or less commonly caloriesmole. So if you had to calculate the Gibbs free energy change at say 298 K you can just slot the numbers in.

Gibbs Free Energy Of Mixing Youtube
17 2 The Gibbs Free Energy And Cell Voltage Chemistry Libretexts

Tang 01b Enthalpy Entropy And Gibb S Free Energy
Kwok The Chem Teacher Chemical Energetics Applying Gibbs Free Energy

Chapter 17 Thermodynamics Spontaneity Entropy And Free Energy General Chemistry An Integrated Approach Hill Petrucci 4 Th Edition Mark P Heitz State Ppt Download

6 2 Potential Kinetic Free And Activation Energy Texas Gateway
Entropy Free Energy And Equilibrium Chapter 18 Thermodynamics

New Chm 152 Unit 6 Power Points Sp13
Solved Unit 13 6 The Standard Gibbs Free Energy Change F Chegg Com

Gibbs Free Energy Dg Dh Tds Chad S Prep

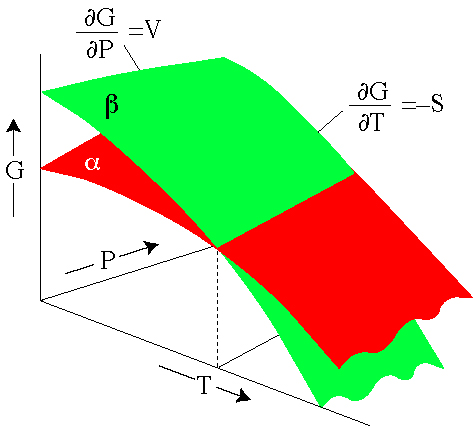
Ppt Pressure Dependence Of Gibbs Free Energy Powerpoint Presentation Id 277495

Unit 11 Chp 5 8 19 Thermodynamics H S G K Ppt Video Online Download
Chemical Potential Enthalpy H Entropy S And Gibbs
Chapter 13 Principles Of Bioenergetics
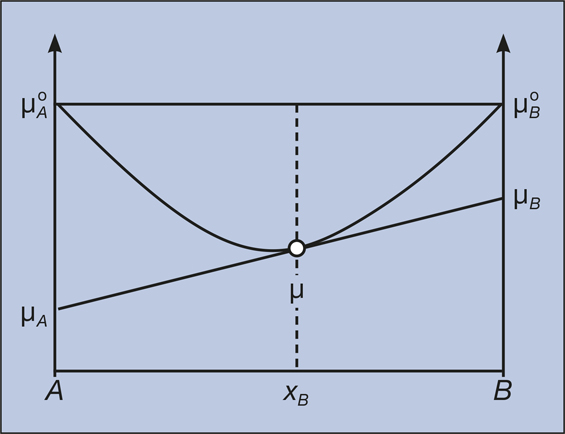
Chemical Potential And Gibbs Free Energy Mrs Bulletin Cambridge Core

Helmholtz Free Energy An Overview Sciencedirect Topics

Heat Transfer And Change In Entropy Gibbs Free Energy Ppt Download

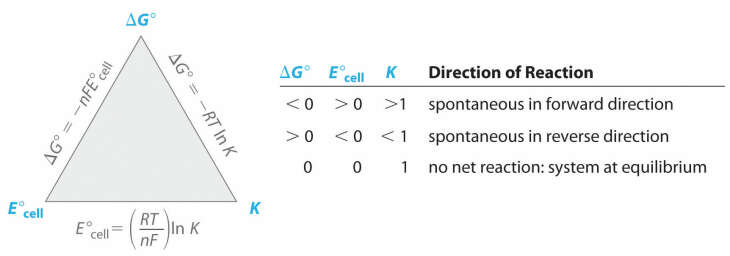
The Relationship Between Free Energy And The Equilibrium Constant Video Lesson Transcript Study Com
Gibbs Free Energy G See Pages 13 15

New Chm 152 Unit 6 Power Points Sp13

Standard Gibbs Free Energy Changes Ag At 25 And 55 C For Reactions Download Table

Question Video Standard Free Energy Change For Hydrogenation Of Ethene Nagwa
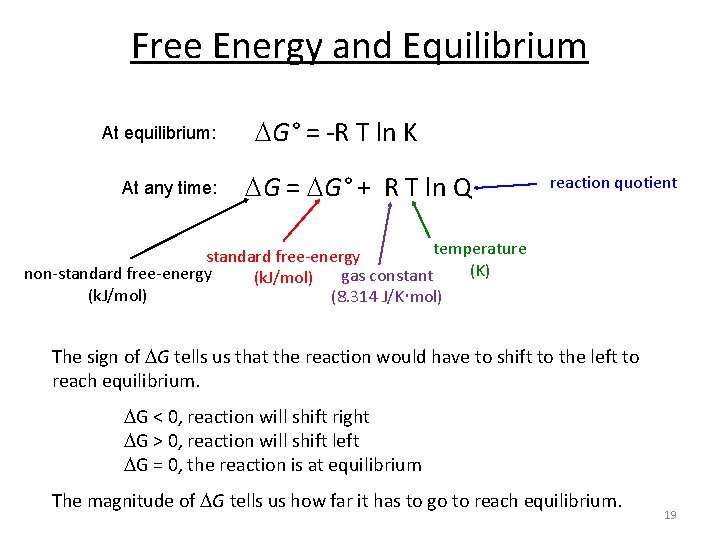
Chapter 17 1 Gibbs Free Energy For A

Tang 01b Enthalpy Entropy And Gibb S Free Energy Teaching Chemistry Chemistry Lessons Chemistry Classroom
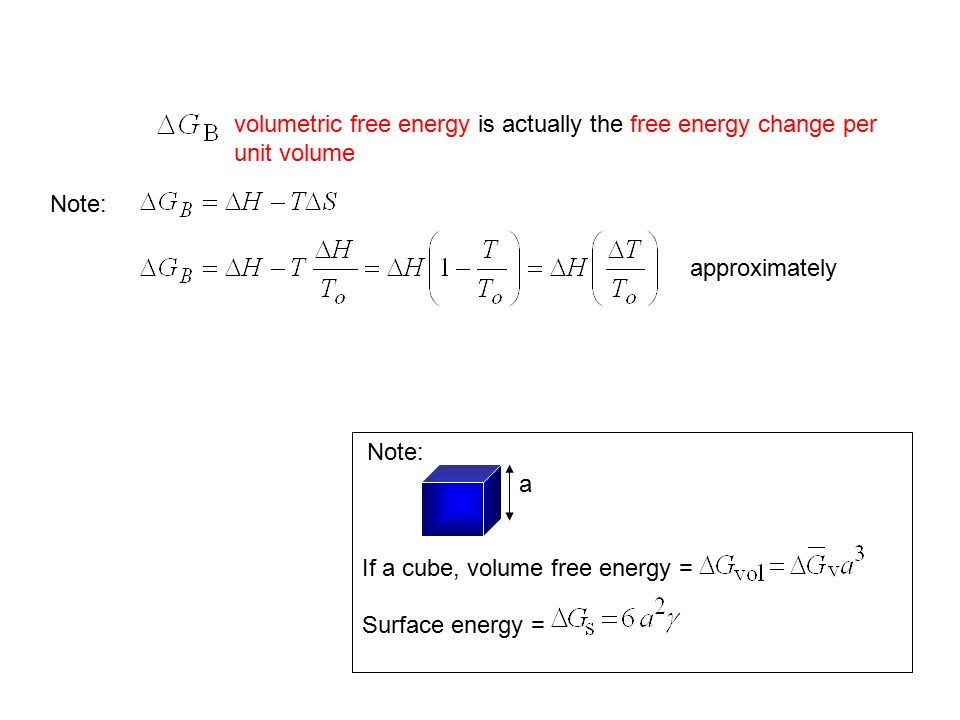
Phase Transformation By Dr Srimala Ppt Video Online Download

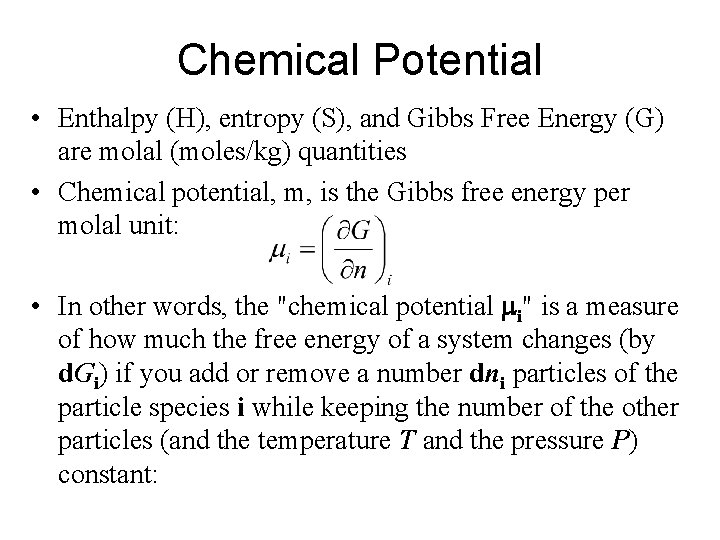
Chemical Potential Combining The First And Second Laws For A Closed System Considering Hence For An Open System That Is One That Can Gain Or Lose Mass Ppt Download
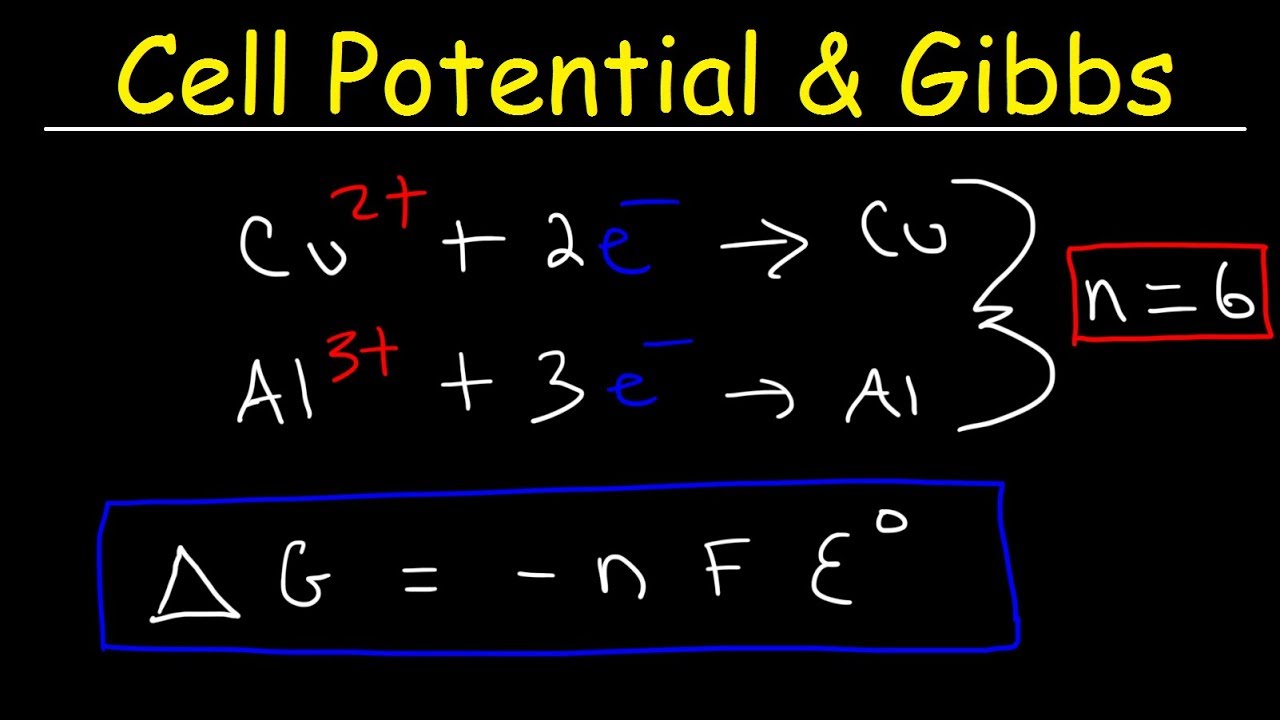
Cell Potential Gibbs Free Energy Standard Reduction Potentials Electrochemistry Problems Youtube

Free Energy And Cell Potential Video Khan Academy
Thermodynamics Spontaneity Of Chemical Reactions Entropy Free Energy
Gibbs Free Energy And Equilibrium Constant Gibbs Free Energy G Is The Thermodynamic Function That Is Most Useful For Biochemistry G Is A Function Of Ppt Download

New Chm 152 Unit 6 Power Points Sp13
Gibbs Free Energy Introductory Chemistry 1st Canadian Edition

The Relationship Between Free Energy And The Equilibrium Constant Video Lesson Transcript Study Com

Heat Transfer And Change In Entropy Gibbs Free Energy Ppt Download
Gibbs Free Energy Gibbs Free Energy Enthalpy

Free Energy Metabolism Metabolic Pathways Flashcards Quizlet
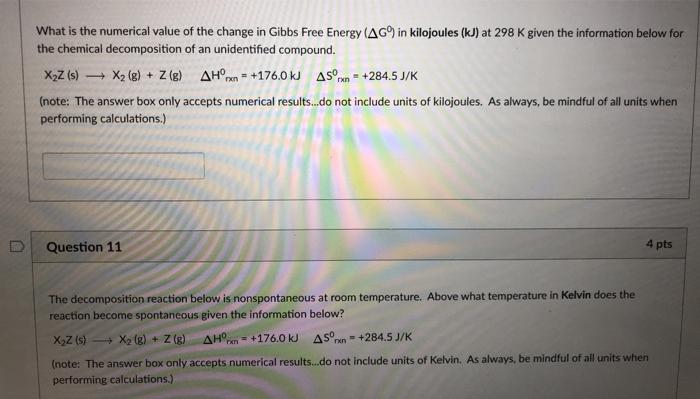
Solved What Is The Numerical Value Of The Change In Gibbs Chegg Com

Gibbs Free Energy Introduction Video Khan Academy
How To Find Equilibrium Constant From Free Energy

Gibbs Free Energy Boundless Chemistry

Gibbs Free Energy Change An Overview Sciencedirect Topics

19 6 Gibbs Energy Change And Equilibrium Chemistry Libretexts
How Is Gibbs Free Energy Related To Enthalpy And Entropy Socratic
O Millesing 2 50 Pts In Chemistry 325 You Will Learn Or Learned That The Standard Gibbs Free Energy Change For A Chemical Reaction Is Course Hero

Gibbs Free Energy Boundless Chemistry

Standard Free Energy Changes Introduction To Chemistry

What Meaning Do Changes In The Absolute Value Of Gibbs Free Energy Have In A Simple Expansion Process Physics Stack Exchange

Physical Chemistry Of Polymers Powerpoint Slides

17 1 Equilibrium And Gibbs Free Energy Hl Youtube

What Will Be Change In Molar Gibbs Free Energy Of H 2 O L At 300 K Constant Temperature If It Is Youtube
Ppt Gibbs Free Energy G Powerpoint Presentation Free Download Id 3325710

Getting Gibbs Energy As A Function Of Temperature
/GettyImages-140670921-5bb4e761c9e77c002644d009-5c2143cf46e0fb0001f993a1.jpg)
What Is Gibbs Free Energy In Chemistry

The Gibbs Free Energy Change Of A Reaction At 27 C Is 26 Kcal And Its Entropy Change Is Youtube

For The Following Reaction The Change In Clutch Prep

The Relationship Between Enthalpy H Free Energy G And Entropy S Video Lesson Transcript Study Com

Kinetics Teach That Electronic Coupling Lowers The Free Energy Change That Accompanies Electron Transfer Pnas

Gibbs Free Energy Of Activation An Overview Sciencedirect Topics

15 2 Gibbs Free Energy Hl Youtube

Gibbs Free Energy Practice Problems Part 1 Youtube
Solved Using The Data In Appendix 4 Determine The Standa Chegg Com

Unit 16 Entropy And Free Energy Youtube

Ppt Gibbs Free Energy Powerpoint Presentation Free Download Id 6125811

Dpy2 Unit 5 Lesson 4 Spontaneity Gibbs Free Energy Equilibrium Lessons Blendspace
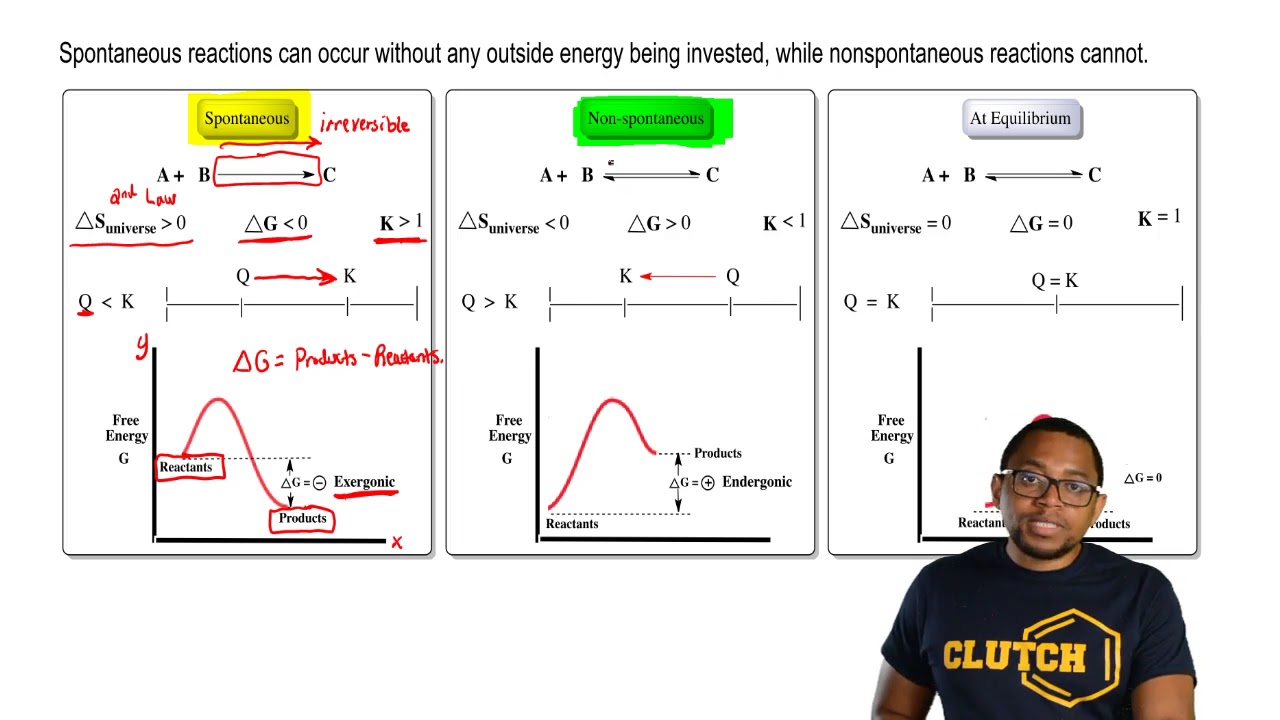
Gibbs Free Energy Chemistry Video Clutch Prep
Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gct7sgsfd7sdex5ds3wvtw9zfebcddxg2cbbn4cemnikv3umnpi8 Usqp Cau

Gibbs Free Energy Example Video Khan Academy
Big Idea 5 Thermochemistry Bond Energy Length Strength Lo 5 1 The Student Is Able To Create Or Use Graphical Representations In Order To Connect The Dependence Of Potential Energy To The Distance Between Atoms And Factors Such As Bond Order

How Can I Calculate Correctly The Gibbs Free Energy Change Of Adsorption Dg
Of The Gibbs Free Energy G Is A Measure Spontane Chegg Com
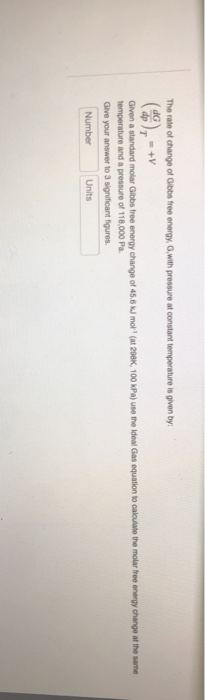
Solved The Rate Of Change Of Gibbs Free Energy G With Pr Chegg Com
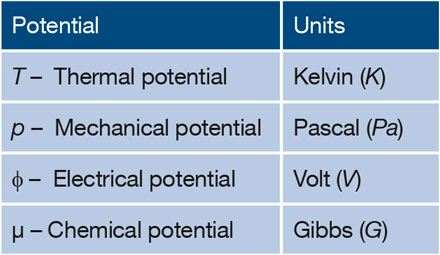
Chemical Potential And Gibbs Free Energy Mrs Bulletin Cambridge Core

6 2 Potential Kinetic Free And Activation Energy Texas Gateway
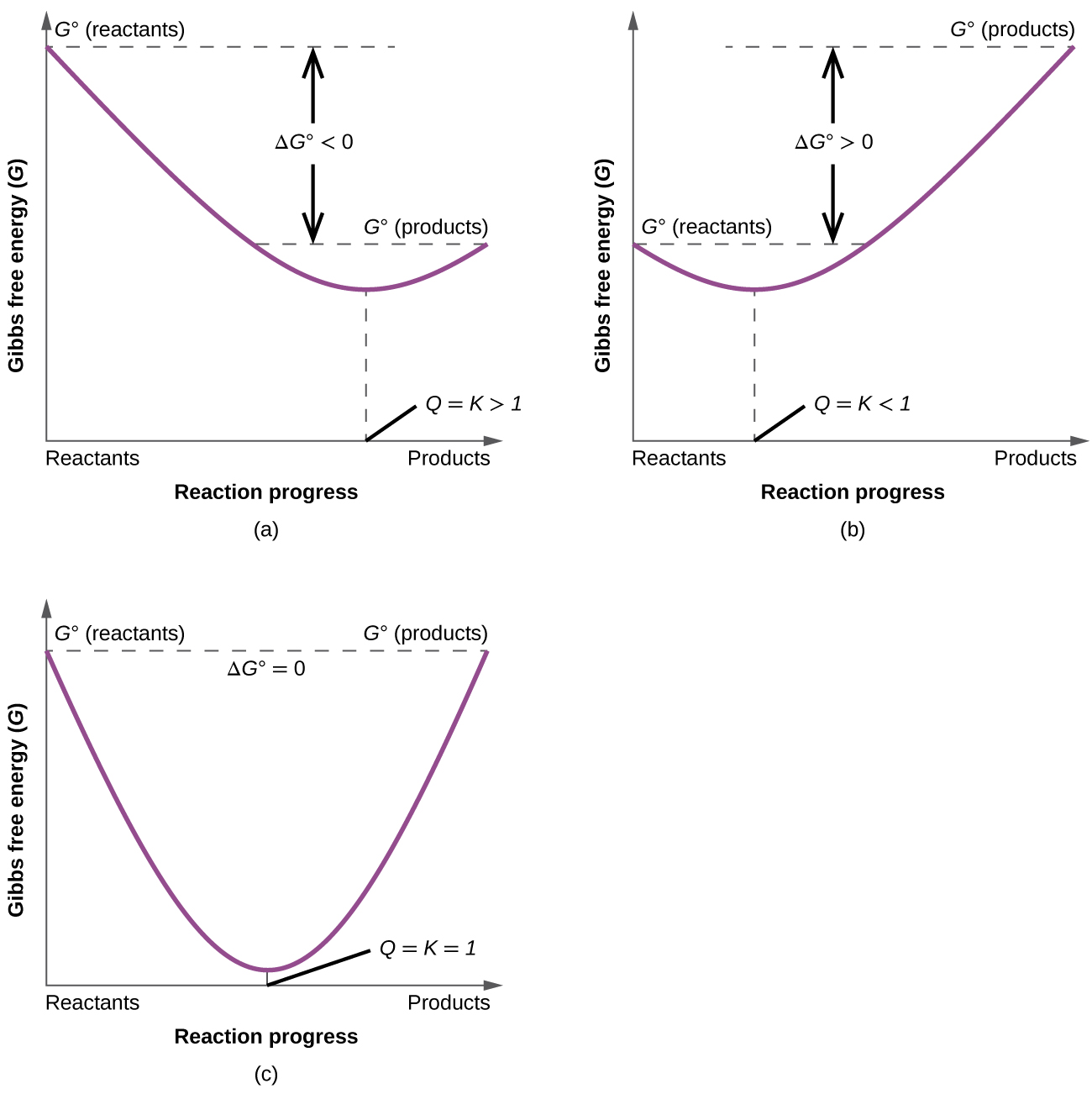
19 6 Gibbs Energy Change And Equilibrium Chemistry Libretexts
Avoiding First Year University Chemistry Textbooks Misrepresentations In The Teaching Of Spontaneous Reactions

Gibbs Free Energy Pressure Dependence Youtube

Sandwalk Better Biochemistry The Free Energy Of Atp Hydrolysis

Derivation Of Gibbs Free Energy Formula Chemistry Stack Exchange
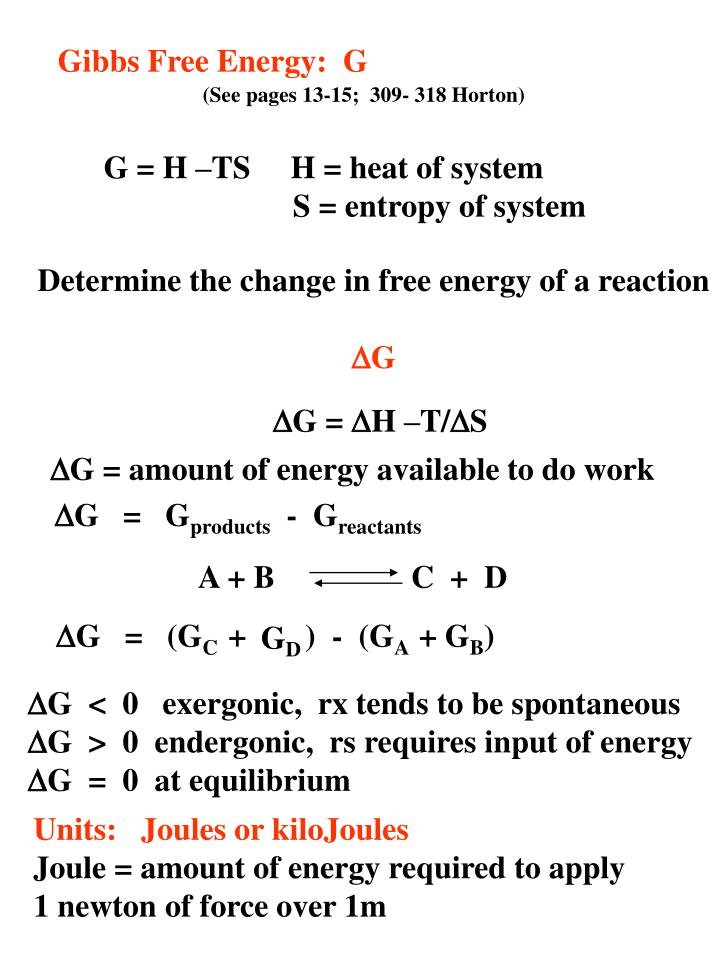
Ppt Gibbs Free Energy G Powerpoint Presentation Free Download Id 3325710

1 The Laws Of Thermodynamics In Review 1 The Internal Energy Of An Isolated System Is Constant There Are Only Two Ways To Change Internal Energy Heat Ppt Download

Chemistry Unit 13 Gibbs Free Energy Homework Pages Store Science And Math With Mrs Lau

Relationship Between Cell Potential And Gibbs Free Energy Change Youtube
Chnage Of Standard Gibbs Free Energy For The Decomposition Of Sodium Hydrogen Carbonate Gibbs Free Energy Enthalpy

Reaction Enthalpy And Change Of Gibbs Free Energy As A Function Of Download Scientific Diagram